


The Influence of Incubation Temperature on Sex Determination in the Veiled Chameleon, Chamaeleo calyptratus
By Jamie Long
Citation:
Long, J. (2008). The influence of incubation temperature on sex determination in the Veiled Chameleon, Chamaeleo calyptratus. Chameleons! Online E-Zine, February 2008. (http://www.chameleonnews.com/08FebLong.html)
Jamie Long
Independent Study
Fall 06/Spring 07
Dr. Despo
Abstract:
The sex of an organism can be determined by a variety of mechanisms, depending on its species. Commonly, sex is fixed at conception by sex chromosomes through genetic sex determination (GSD). However, there are many species in which environmental factors influence the differentiation of bipotential gonads into either ovaries or testes as the embryo develops. Incubation temperature plays a vital role in sex-ratio outcomes of many animals, especially reptiles, by what is known as temperature-dependent sex determination (TSD). During TSD, temperature affects steroid metabolism of the embryo within the egg, eventually establishing sex irreversibly part way through development. It was the intention of this study to demonstrate whether or not the Veiled Chameleon, Chamaeleo calyptratus, is subject to the mechanism of TSD. Seven clutches of chameleon eggs were divided into three groups and incubated at one of three carefully controlled temperatures, 29, 26.5 and 24.5°C, throughout the duration of their 6 to 9 month development period until hatching. Sex data from over 350 live hatchlings was included in the study, and an overall mortality rate of less than 2% assured that failure of eggs to hatch did not affect the final results. The length of incubation time was significantly impacted by temperature, with lower temperatures increasing the duration of development. However, because there was no statistical difference, according to the Chi-squared analysis, between the sex ratios of the three groups, the study suggests that the sex of Chamaeleo calyptratus is not governed by incubation temperature, but most likely fixed at conception by genetic factors.
Introduction:
It is possible for the sex of an organism to be determined in a number of different ways. It may be set at the time of conception through what is known as genetic sex determination (GSD) or later by environmental factors like temperature. The discovery of temperature-dependent sex determination (TSD) was credited to Madeline Charnier of Senegal in 1966 (Valenzuela and Lance 2004). Since, the TSD mechanism has been studied in various reptiles, amphibians and fish. According to D.C. Deeming, all species of crocodilians, many species of turtles and squamates, and a few fish exhibit TSD (2004). However, not all species that show evidence of TSD are affected in the same way. Importantly, three different categories of TSD have been described. The first class is referred to as TSD Ia, in which low incubation temperatures produce males and higher temperatures produce females. TSD Ib is just the opposite, with low temperatures making females and high ones making males. TSD II is slightly different, in that at both low and high temperatures females are produced and males arise only at an intermediate temperature.
Each species subject to TSD has a relatively brief stage, usually at some point in the middle third of embryonic development, known as the thermosensitive period (TSP). The TSP, which can account for 18-30% of development, is the time during when incubation temperature affects sex (Pieau and others 1999; Valenzuela and Lance 2004). Different species have their own unique incubation requirements as well as specific pivotal temperatures that influence sex ratios.
It is important to realize that, in a natural setting there may be continual temperature variation at a nest site. Seasonal and daily temperature fluctuations, temperature gradient within nests, as well as weather conditions like wind and precipitation may potentially alter developmental outcomes (Valenzuela and Lance 2004). While, for many species, the pivotal temperatures that determine sex are known with relative certainty; it remains to be proven exactly how much time during the thermosensitive period must be spent above or below that critical temperature to produce irreversible sex determination.
The process by which temperature influences developing embryos, establishing physiological and physical characteristics, in order to delineate sex is not yet fully understood. It is also unclear whether all species subject to TSD are influenced in the same ways. It has become apparent that temperature affects embryos sex steroid metabolism during development. Most researchers agree that the level of aromatase, an enzyme that converts androgens to estrogens, is regulated by incubation temperature (Pieau and Dorizzi 2004). Those steroids, in turn, influence the differentiation of bipotential or indifferent gonads into either ovaries or testes. By the end of the thermosensitive period (TSP) sex had been established, and some reproductive cells have typically begun meiosis. While a recent study suggests that temperature levels solely manipulate gonadal tissue, others have surmised that extragonadal tissues, such as adrenal, brain, and yolk may be affected and have an impact on the organism's sex as well (Pieau and Dorizzi 2004). Also, it has been found that the TSD mechanism can be superseded by the topical application of steroids, steroid inhibitors, or steroid mimics to developing eggs.
Along with sex, temperature during incubation may also affect rate of embryonic development, length of incubation period, yolk reserves remaining upon hatching, hatchling size and morphology, coloration, post-hatching behavior, and growth (Deeming 2004; Valenzuela and others; 2003) . However, these factors further complicate the matter. While temperature may have obvious effects on the sex-ratio outcome of a species; that is not enough evidence to assure TSD. GSD + Environmental Effect occurs when environmental factors such as temperature override sex chromosomes. This category includes sex reversal, differential mortality, and differential fertilization, meaning that exogenous cues either prevent development of certain zygotes, prevent survival/vitality of particular gametes, or cause physiological changes in an organism not normally associated with its genetic sex (Valenzuela and others 2003). Therefore, even if sex-ratio bias is associated with temperature, further study must be conducted to differentiated between the TSD and GSD+ environmental effect mechanisms.
There is no definitive evidence to suggest whether TSD or GSD evolved first if one arose from the other. However, it is important to remember that TSD does not imply a lack of genetic involvement. TSD simply displays a lack of heteromorphic sex chromosomes or temperature insensitive determination factors. It is also evident that TSD is exhibited in many old and long-lived species such as crocodiles and turtles. Therefore, the success of this mechanism is obvious. Nevertheless, it remains to be seen how human influence or a global climate change may affect TSD species (Janzen 1994).
While the mechanism of TSD and its evolutionary significance are not completely understood, the full extent of TSD remains undetermined because many taxa have not been studied. According to Valenzuela and Lance, there are no confirmed cases of TSD in chameleons, but there are many anecdotal accounts of it (2004). Therefore, more studies are necessary in this area. My research is intended to determine the mode of sex determination of one species of chameleon, Chamaeleo calyptratus, the Veiled Chameleon.
Materials & Methods:
I obtained three identical "Standard View Thermal Air Flow Hova-bator Reptile Incubators" from the Randall Burkey Company of Boerne, TX 78006. These incubators were set up side by side in a location where they would not be disturbed. Each of the three hova-bators was programmed to maintain a different temperature inside, 29°C, 26.5°C, and 24.5°C respectively. Generally, reptile eggs can tolerate a temperature fluctuation of 5-8°C (Deeming 2004). The chosen temperature range encompasses the majority of the viable incubation range for veiled chameleons (Schmidt and others 1994). The incubators equilibrated to room conditions for no less than a week, and careful monitoring with a mercury thermometer ensured that temperatures inside were stable at the proper level prior to the acquisition of any eggs.
I obtained 407 Chamaeleo calyptratus eggs from reptile breeder Craig Long, of Home Pennsylvania, 15747. Clutches from a variety of females sired by various males were used in the experiment to reduce the potential influence of specific individuals. Gravid females were allowed to nest in a box of damp sand, and after laying the eggs and completing the nesting ritual females were removed from the box. Each clutch of eggs was collected less than 12 hours after being laid. Most clutches were divided into three equal parts, one to be incubated at each of the three temperatures.
Incubation substrate was created by mixing two cups hydrated laminar magnesium-aluminum-ironsilicate, commonly known as vermiculite, with 3/8 cup distilled water. Rubbermaid Takealongs plastic containers were filled half way with a layer of substrate. Then each egg was gently bedded into the medium leaving the top third exposed. All containers had two pinholes placed in the sides to allow airflow, and were then covered with a tight fitting lid. Each container, holding anywhere from 16 to 32 eggs, was labeled with information such as date laid and parent names.
I immediately placed the containers into the incubators. Each then remained inside its respective incubator until the eggs within had hatched. However, the containers were periodically opened, at least once every 2 weeks, to allow fresh air in as well as to check the moisture of the vermiculite. Distilled water was added with a spray bottle if dryness appeared, but eggs were never directly sprayed with water.
As the eggs matured, between 170 and 252 days after being laid, I examined them every other day for signs of hatching. Once hatchlings emerged, I removed them from the containers and immediately sexed them based on the presence or absence of heel spurs. Male veiled chameleons exhibit a tiny tab of tissue on the posterior of each back foot, whereas females do not (Figure 1). This spur is evident at birth and persists throughout life. Observations were made about each group of hatchlings including sex-ratio which was documented and analyzed statistically using a Chi-squared test.
Results:
Eggs for this experiment were laid between 4/24/06 and 7/17/06. A total of 407 eggs were included in the experiment; however, 6 eggs failed to hatch. A mortality rate of 1.5% was observed, allowing 401of the total eggs to produce viable hatchlings.
The amount of time required for egg development varied greatly between the three groups. Eggs hatched between 10/11/06 and 3/21/07, anywhere from 170 to 271 days after being laid, with the warmer temperature producing the shortest incubation time and the coolest temperature, the longest. At 29°C eggs required an average of 185 days to hatch. At 26.5°C they averaged 219 days and 252 days at 24.5°C. A simple analysis of variance (ANOVA) test confirmed that incubation time was significantly different between the three groups (F=26.6, P<.05). A Tukey's Test verified that length of incubation was significantly different for all three groups.
Overall, 51.6% of hatchlings were female and 48.4% male. The intermediate incubation temperature produced slightly more males than females, while the others were female biased. However, all sex ratios (Table 1) remained nearly 1:1.
Table 1- Sex of Hatchlings Produced at Each Incubation Temperature
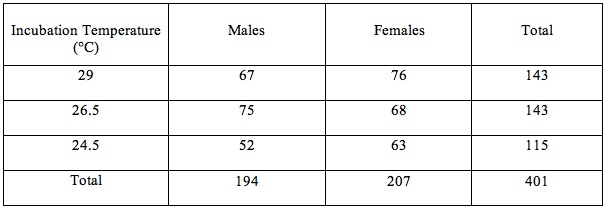
Sex ratio data (Table 1) was compared using a Chi-squared analysis (X^2= 1.54, df=2, P<.05). Therefore, the null-hypothesis, that the sex ratios are the same among all groups, is accepted. Unhatched eggs were not considered when performing this statistical analysis.
Discussion:
The Veiled Chameleon is native to areas of Yemen and Saudi Arabia. They are commonly found in both tropical forest areas with high humidity and drier desert regions (Andrew and Donoghue 2004). These Chameleons are adept tree-dwellers with prehensile tails and specialized feet that grip branches. Their unique ability to change color affords them some measure of camouflage; however, it is most commonly used to display mood and can deter predators such as birds and snakes.
Data collected during this experiment suggest that anecdotal accounts of TSD in the Chamaeleo calyptratus species are unsubstantiated. Each of the eggs was subjected to a single temperature throughout its entire incubation period with very minimal fluctuation. Therefore, all eggs would have, undoubtedly, spent the duration of their thermosensitive period (if they are subject to one) at one of the three temperatures. A range of about 5°C was chosen to ensure low mortality rates. Incubation temperatures over 30°C tend to produce low hatch rates and/or very weak hatchlings (De Vosjoli and Ferguson 1995; Andrews 2005). Because most of the viable range of incubation temperatures for the veiled chameleon was included in the experiment, if TSD occurred in this species, it ought to have been exhibited in the sex-ratio of the hatchlings. However, after comparing sex ratios at all temperatures, no significant differences existed.
Egg mortality, or failure to hatch, was extremely low. The mortality rate of less than 1.5% would have no significant influence on the final outcome of the experiment, regardless of the sex of the embryos.
This experiment did show that incubation temperature does influence aspects of development in the veiled chameleon other than sex. For instance, increased incubation temperature corresponded with shorter incubation time, suggesting that higher temperatures increase the rate of embryonic development within the egg. However, hatchlings from all temperatures emerged with very similar morphology.
Increased incubation period may have some negative consequences in a natural environment. Veiled Chameleons bury each clutch of eggs under damp sand or soil at a single location (Schmidt 1994). The longer eggs remain in the nest the longer they would be subject to disturbance, predation, changes in weather, and microorganisms such as fungus. Disruption of the eggs is likely to result in mortality. Once the eggs harden after being laid, turning them will cause the embryos to become disconnected from the inner shell surface and die, a fate comparable to placental abruption in mammals (Deeming 2004). Lengthening the incubation period allows more time for outside factors to interfere with the nest causing survival rates to decrease.
It was noted that the chameleons that hatched in from eggs in the 29°C incubation were much more active, immediately post hatching, than hatchlings from either the 26.5°C or 24.5°C temperatures. In addition, the individuals from the higher temperature had a greater appetite immediately after hatching than those of the other two groups. Hatchlings from the warmest temperature began consuming crickets within hours of hatching, whereas the others started to eat after 12-24 hours. This may indicate that higher incubation temperature results increased metabolism at the time of hatching. However, this was not tested by any objective means.
After hatching, all chameleons were reared in similar habitats under nearly identical conditions. The chameleons were observed in a general way for about five weeks post hatching. During that time, growth rates, food consumption, and activity level were similar between all groups, and no hatchling mortality was observed. There did not appear to be any persistent developmental influence of their incubation temperature.
Works Cited
Andrews RM. 2005. Incubation Temperature and Sex Ratio of the Veiled Chameleon (Chamaeleo calyptratus). Journal of Herpetology. 39.3: 515-8.
Andrews RM, Donoghue S. 2004. Effects of Temperature and Moisture on Embryonic Diapause of the Veiled Chameleon (Chamaeleo calyptratus). Journal of Experimental Zoology. 30: 629-35.
Deeming, DC, editor. 2004. Reptilian Incubation Environment, Evolution and Behavior. Nottingham, UK: Nottingham University Press. 362p.
De Vosjoli P and Ferguson G, editors. 1995. Care and Breeding of Panther, Jackson’s, Veiled, and Parson’s Chameleons. Santee: Advanced Vivarium Systems Inc. 128p.
Janzen FJ. 1994. Climate change and temperature-dependent sex determination. Proceedings of the National Academy of Sciences of the United States of America. 91: 7487-7490.
Pieau C, Dorizzi M, Richard-Mercier N. 1999. Temperature-dependent sex determination and gonadal differentiation in reptiles. Cellular and Molecular Life Sciences. 55: 887-900.
Pieau C, Dorizzi M. 2004. Oestrogens and temperature-dependent sex determination in reptiles: all is in the gonads. Journal of Endocrinology. 181: 367-77.
Schmidt W, Tamm K, Wallikewitz E. 1994. Chameleons Volume II Care & Breeding. Neptune City: T.F.H Publications Inc. 64p.
Valenzuela N, Lance V, editors. 2004. Temperature-Dependent Sex Determination in Vertebrates. Washington: Smithsonian Books. 200p.
Valenzuela N, Adams DC, Janzen FJ. 2003. Pattern Does Not Equal Process: Exactly When Is Sex Environmentally Determined? The American Naturalist. 161: 676-83.
Figure 1

Above: Adult male chameleon

Jamie Long

Jamie Long, a 2007 graduate of Thiel College, is currently continuing her education at Case Western School of Dental Medicine. Always a wildlife hobbyist, Jamie became a full-fledged chameleon enthusiast after acquiring her first chameleon "Snickles" in early 1999. Since then, she has played an integral role in the development of her family's chameleon breeding business Total-e-Chamz. Although attending college required her to leave the chameleon ranch, it did afford Jamie the opportunity to pursue research into the life of these fascinating reptiles.









Join Our Facebook Page for Updates on New Issues:

© 2002-2014 Chameleonnews.com All rights reserved.
Reproduction in whole or part expressly forbidden without permission from the publisher. For permission, please contact the editor at editor@chameleonnews.com
